
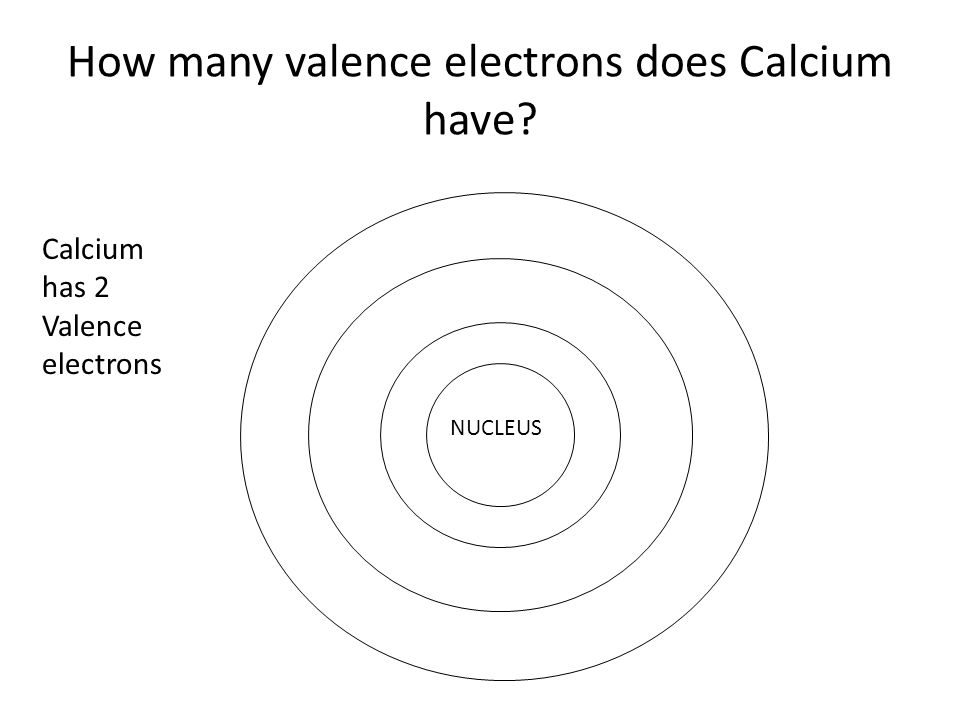
Similarly, the atoms of all group 7 elements have similar chemical properties and reactions to each other because all of them have seven electrons in their outer shell. Explanation: Calcium has an atomic number of 20. The atoms of all group 1 elements have similar chemical properties and reactions because they all have one electron in their outer shell. The electronic configurations of the elements in group 1: So, elements in the same group have similar chemical properties because they have the same number of electrons in their outer shell. When atoms collide and react, it is the outer electrons that meet and interact. The electronic configurations of atoms help explain the properties of elements and the structure of the periodic table. has an atomic number of (2 + 8 + 1) = 11Įlectronic configurations and properties of elements.The electronic configuration of sodium (2.8.1) shows that sodium, Na: the number of electrons in all shells of an element is represented in the periodic table as the element's atomic number Ca 2e Ca 2+ The electron configuration of calcium ion(Ca 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6.How many valence electrons does Calcium have The. configuration Ne3s1 of elements A, B and Care He2s1, and Ar4s1. Element with electron configuration 1s22s22p63s23p64s2 is Calcium (Ca) that has the atomic number of 20. the number of electrons in the outermost shell of an element is represented in the periodic table as the group number that element is situated in electron will break this stable electronic configuration and requires high.the number of circles in the electronic configuration of an element is represented in the periodic table as the period number that element is situated in Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game.The electronic configuration of an element is related to its position on the periodic table. Those tiny little subatomic particles, whizzing around in their orbits.Electronic configurations and the periodic table
Ca element electron configuration full#
1s2 2s2 2p6 3s2 4p6 Write the full electron configuration for strontium (Z 38). Write the electron configuration for the element chlorine.

Application of Le Chatelier's Principle.Structure, Composition & Properties of Metals and Alloys.Intramolecular Force and Potential Energy.When we write the configuration we'll put all 20 electrons in orbitals around the nucleus of the Calcium atom. Variable Oxidation State of Transition Elements In order to write the Calcium electron configuration we first need to know the number of electrons for the Ca atom (there are 20 electrons).Transition Metal Ions in Aqueous Solution.Single and Double Replacement Reactions.


 0 kommentar(er)
0 kommentar(er)
